 August is Children’s Eye Health and Safety Month, a critical time to prioritize the vision of our youngest patients. Clutter and disorganized storage in healthcare settings can pose significant risks to children’s eye safety, including accidental injuries and exposure to harmful substances. At Storage Systems Unlimited (SSU), we are committed to equipping healthcare facilities with the best storage solutions to foster safe and organized environments.
August is Children’s Eye Health and Safety Month, a critical time to prioritize the vision of our youngest patients. Clutter and disorganized storage in healthcare settings can pose significant risks to children’s eye safety, including accidental injuries and exposure to harmful substances. At Storage Systems Unlimited (SSU), we are committed to equipping healthcare facilities with the best storage solutions to foster safe and organized environments.
Protecting Our Future Vision:
For pediatric healthcare professionals, safeguarding young eyes is vital. Your proactive approach in protecting young patients’ eye health and safety makes a substantial difference. SSU offers a range of storage solutions tailored to the unique needs of pediatric ophthalmic practices.
Protecting Little Eyes with Our Big Solutions:
A well-organized and hazard-free environment is essential for children’s health and safety. Our storage solutions are designed to maximize space and secure supplies, minimizing the risk of exposure to hazards, and promoting optimal eye health for children.
Our Storage Solutions:
SSU provides safe, efficient, and organized storage solutions for pediatric facilities. Our products are built with durability and child safety in mind. We offer:
- Bins and Containers: Made from polypropylene material to withstand autoclave sterilization, these bins help mitigate contamination risks.
- Secure Cabinets with Antimicrobial Coatings: Safeguard medical supplies and prevent hazardous materials exposure.
- High-Density Shelving: Streamline workflow with easy access to supplies. Maximize space with easy-to-clean, microbial-resistant surfaces.
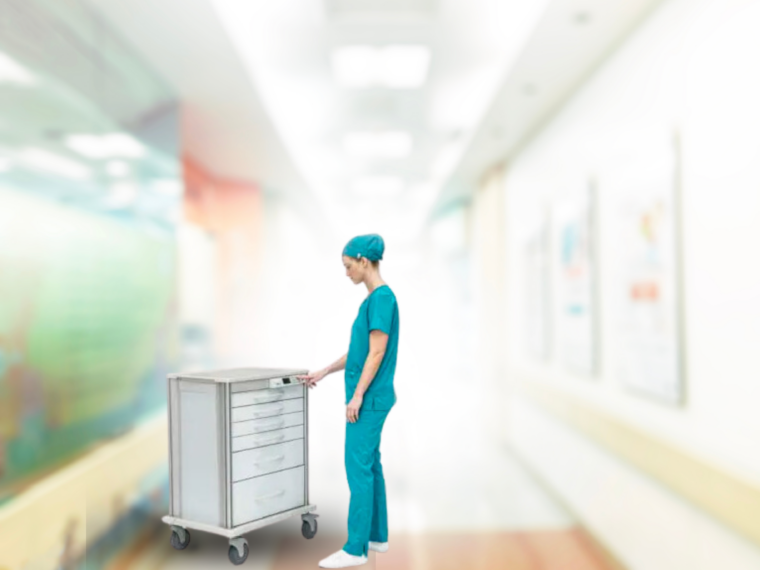
- Medical Supply Carts: Efficiently transport, organize, and secure supplies with customizable lock options. Antimicrobial surfaces further enhance infection prevention efforts.
- Secure IntraOcular Lens Carts: Protect the integrity of young patients’ lens implants.
- Patient Room Casework and Furniture: Maintain an organized and safe patient room.
- Chemical Storage Cabinets: Designed to meet strict safety standards.
By optimizing your storage space and ensuring the safety of your equipment and supplies, you create a secure and welcoming environment for young patients and their families.
Create a safer, more organized healthcare facility this Children’s Eye Health and Safety Month. Contact us today at 888-614-0004 to discover how our storage solutions support the eye health and safety of your young patients.
Let’s unite to protect the precious gift of sight in our children. Together, we can shape a healthier, brighter future for all pediatric patients.